Product Details
Appearance:light yellow powder ,insoluble in water
Trustworthy Quality Manufacturer Supply Best Quality 59870-68-7 with Fast & Safe Shipping
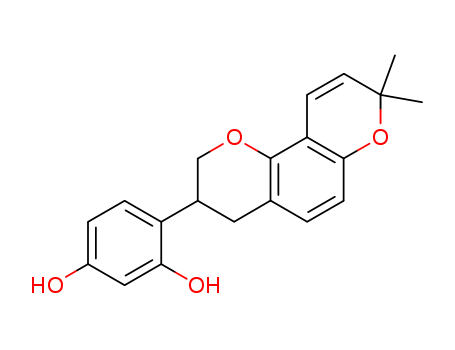
- Molecular Formula:C20H20O4
- Molecular Weight:324.376
- Appearance/Colour:light yellow powder ,insoluble in water
- Vapor Pressure:2.26E-11mmHg at 25°C
- Melting Point:154-155oC
- Refractive Index:1.622
- Boiling Point:518.555 °C at 760 mmHg
- PKA:9.66±0.40(Predicted)
- Flash Point:267.413 °C
- PSA:58.92000
- Density:1.257 g/cm3
- LogP:4.00070
Glabridin(Cas 59870-68-7) Usage
|
Biochem/physiol Actions |
Glabridin is a bio-available isoflavan isolated from licorice (Glycyrrhiza glabra L.) root extract. Glabridin has been reported to posses varies biological activities including strong antioxidant activity, antioxidant against LDL oxidation, anti-Helicobacter pylori properties, estrogen-like activity and antinephritic and radical scavenging activities. Also it appears to inhibit serotonin re-uptake, melanogenesis, inflammation and cytochrome P450 3A4, 2B6, and 2C9. |
|
Definition |
ChEBI: A member of the class of hydroxyisoflavans that is (R)-isoflavan substituted by hydroxy groups at positions 2' and 4' and a 2,2-dimethyl-2H-pyran group across positions 7 and 8 respectively. |
InChI:InChI=1/C20H20O4/c1-20(2)8-7-16-18(24-20)6-3-12-9-13(11-23-19(12)16)15-5-4-14(21)10-17(15)22/h3-8,10,13,21-22H,9,11H2,1-2H3/t13-/m0/s1
59870-68-7 Relevant articles
Method for synthesizing glabridin
-
Paragraph 0031; 0044-0045, (2021/11/14)
The invention relates to a method for sy...
Method for synthesizing optically pure glabridin
-
, (2021/11/19)
The invention relates to a method for sy...
Method for asymmetrically synthesizing glabridin with optical purity
-
, (2020/07/15)
The invention discloses a method for asy...
A synthesis method of glabridin (by machine translation)
-
Paragraph 0024; 0043; 0044, (2019/02/04)
The invention discloses a glabridin synt...
59870-68-7 Process route
-
-
2',4'-dimethylglabridin

-
- 59870-68-7
(±)-glabridin
| Conditions | Yield |
|---|---|
|
2',4'-dimethylglabridin; With boron tribromide; In dichloromethane; at 0 ℃; for 1.5h; Inert atmosphere;
With methanol; In dichloromethane; for 0.5h; Inert atmosphere;
|
100% |
|
With boron tribromide; In dichloromethane; at -78 - 20 ℃; for 2h;
|
84% |
|
With boron tribromide; In dichloromethane; at -78 ℃; for 2h; Inert atmosphere;
|
79% |
-
-
2',4'-di(methoxymethyl)glabridin

-
- 59870-68-7
(±)-glabridin
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; isopropyl alcohol; at 20 ℃; for 5h;
|
59870-68-7 Upstream products
-
151-10-0
1,3-Dimethoxybenzene
-
829-20-9
2',4'-dimethoxyacetophenone
-
108-46-3
recorcinol
-
6496-89-5
2,4-dimethoxyphenylacetic acid
59870-68-7 Downstream products
-
59870-70-1
(R)-3-(2,4-dimethoxyphenyl)-8,8-dimethyl-3,4-dihydro-2H,8H-pyrano[2,3-f]chromene

