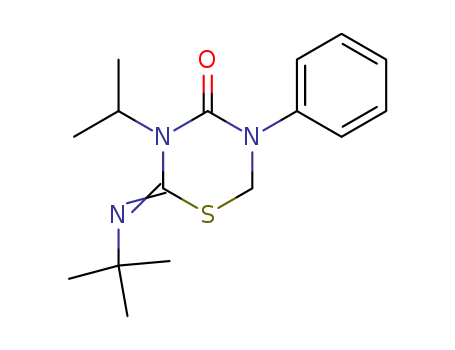
69327-76-0
- Product Name:Buprofezin
- Molecular Formula:C16H23N3OS
- Purity:99%
- Molecular Weight:
Product Details
Appearance:white to pale yellow crystalline powder
69327-76-0 Properties
- Molecular Formula:C16H23N3OS
- Molecular Weight:305.444
- Appearance/Colour:white to pale yellow crystalline powder
- Vapor Pressure:8.12E-09mmHg at 25°C
- Melting Point:104-106 °C
- Refractive Index:1.52-1.522
- Boiling Point:395 °C at 760 mmHg
- PKA:3.02±0.20(Predicted)
- Flash Point:192.7 °C
- PSA:61.21000
- Density:1.12 g/cm3
- LogP:4.18510
69327-76-0 Usage
Uses
Buprofezin is a insecticide which works as a chitin synthesis inhibitor. Buprofezin is used to control stubborn rice pests and is effective in controlling green leaf hoppers and white back plant hoppe rs for long durations.
Uses
Insecticide.
Uses
Buprofezin is a contact and ingested insecticide, active against Homoptera (whiteflies, leafhoppers, scale insects, etc.) in and on citrus, cotton, cucumbers, tomatoes, sweet potatoes, rice, etc.
Hazard
Moderately toxic by ingestion. Low toxic- ity by skin contact.
Agricultural Uses
Insecticide, Acaricide, Insect growth regulator: For insect control in food crops and greenhouse ornamentals.
Trade name
APPLAUD?; NNI-750?
Pharmacology
The foregoing indicates that the modes of action of buprofezin and benzoylureas could be similar or identical. However, one differing biochemical effect of buprofezin is inhibition of prostaglandin biosynthesis (33), a mechanism that has been suggested as responsible for its ovicidal activity. Subsequently, the in vitro and in vivo effects of buprofezin were found to be strongly antagonized by 20- hydroxyecdysone (34), which also affected prostaglandin biosynthesis. Thus, inhibition of both prostaglandin and chitin biosynthesis by buprofezin was prevented by 20- hydroxyecdysone, so that both effects of the insecticide are mediated via an effect on the hormone concentration or its receptor. Consequently, buprofezin seems to inhibit the drop in the 20-hydroxyecdysone titer that triggers epidermal cell proliferation, old cuticle digestion, and new cuticle deposition, but the detailed mechanism of this action has yet to be established.
Metabolic pathway
Buprofezin gradually decomposes in soils under flooded and upland conditions, with half-lives of 104 and 80 days, respectively. After 150 days, five degradation products are identified as 2-tert- butylimino-5-(4-hydroxyphenyl)-3-isopropylperhydro- 1,3,5-thiadiazin-4-one, 3-isopropyl-5-phenylperhydro- 1,3,5-thiadiazin-2,4-dione, 1-tert-butyl-3-ispropyl-5- phenylbiuret, 1-isopropyl-3-phenylurea, and phenylurea. As minor products, 2-tert-butylimino-5- phenylperhydro-1,3,5-thiadiazin-4-one or buprofezin sulfoxide are found in the flooded or in the upland soils. Since neither formation of 14CO2 nor hydroxylation is observed in the sterile soils, buprofezin seems to have undergone complete mineralization in soils under both conditions through biological transformation by soil microorganisms.
Degradation
Buprofezin (1) was degraded under acidic conditions with half-lives (DT50) f 6-12 days (pH 4), 34 days (pH 6) and 65 days (pH 10) at 40 °C. Opening of the thiadiazinanone ring appeared to be the primary hydrolytic degradation pathway, to yield 1-tert-butyl-3-isopropyl-5-phenyl-2- thiobiuret (2) and N-isopropyl-N-phenylurea (3) as major products. Buprofezin is stable to aqueous photolysis. The estimated DT50 of buprofezin in distilled water when exposed to UV light was 39 days. A more complex photodegradation pathway of buprofezin in methanol was reported recently (Datta and Walia, 1997) with DT9 values of 4 hours and 15 days under UV and sunlight irradiation, respectively.
InChI:InChI=1/C16H23N3O2S/c1-11(2)19-14(17-16(3,4)5)22-10-18(15(19)21)12-6-8-13(20)9-7-12/h6-9,11,20H,10H2,1-5H3/b17-14-
69327-76-0 Relevant articles
Method for continuously synthesizing buprofezin
-
Paragraph 0043-0061, (2020/12/30)
The invention discloses a method for continuously synthesizing buprofezin, and belongs to the technical field of chemical synthesis. The method for continuously synthesizing buprofezin comprises the following steps: S1, continuously acylating N-methylaniline and phosgene in a solvent, and continuously deacidifying to obtain an acyl chloride solution; S2, carrying out continuous chlorination and continuous deacidification on the acyl chloride solution and chlorine under the action of an initiator to obtain a chloride solution; S3, adding buprothiourea, a catalyst and an acid-binding agent intothe chloride solution, carrying out continuous mixing reaction, crystallizing and drying a reaction product to obtain buprofezin of which the content is greater than or equal to 99.0% and the total yield is greater than or equal to 88% based on N-methylaniline. The method for continuously synthesizing buprofezin solves the defects caused by batch production in China, has the advantages of continuous reaction, high automation degree, small equipment size, high operation safety, few side reaction products, high conversion rate and good product quality, can effectively improve the production efficiency and reduce the production cost, and has very good popularization and application values.
Synthesis method of buprofezin
-
Paragraph 0045; 0054-0081, (2019/08/03)
The invention provides a synthesis method of buprofezin. The synthesis method comprises the following step: carrying out a cyclization reaction on raw materials including N-chloromethyl-N-phenylaminoformyl chloride and N-tert-butyl-N'-isopropylthiourea, wherein an acid binding agent takes acetate as a main component. According to the synthesis method provided by the invention, sodium acetate is adopted to replace ammonium bicarbonate and is used as alkali needed for synthesizing the buprofezin; the problem that solvent loss is caused by the release of carbon dioxide generated in a process of synthesizing a product through the ammonium bicarbonate is solved, and a byproduct, namely sodium chloride, can be directly filtered and removed and can be recycled to obtain certain economic benefit;meanwhile, energy consumption generated by wastewater treatment is reduced and zero emission of wastewater is realized; the average yield of the buprofezin prepared by adopting the synthesis method provided by the invention reaches 86 percent or more, and the content of the finished-product buprofezin reaches 97.0 percent or more.
Preparation method of buprofezin
-
, (2018/10/11)
The invention provides a preparation method of buprofezin, comprising the steps of 1), adding sodium thiocyanate and water into an esterification kettle until dissolution; adding tertiary butanol andhydrochloric acid to obtain a mixed ester; allowing transposition and catalytic reaction to obtain t-butyl isothiocyanate; 2), adding the t-butyl isothiocyanate into chlorobenzene, stirring, dropwiseadding isopropyl amine to obtain 1-isopropyl-3-tert-butylthiourea solution; 3), adding N-methylaniline into chlorobenzene; introducing carbonyl chloride and chlorine gas in sequence to obtain N-chloromethyl-N-benzenecarbamoylchloride solution; 4), adding sodium bicarbonate into water, and adding the resultant into chlorobenzene; adding the 1-isopropyl-3-tert-butylthiourea solution; dropwise addingthe N-chloromethyl-N-benzenecarbamoylchloride solution, filtering, allowing layering, performing high-vacuum steaming to remove the chlorobenzene, crystallizing, centrifuging, and drying to obtain buprofezin. The substitutive average yield of the preparation process of buprofezin reaches 90% and above; the content of finished buprofezin reaches 98.0% and above; the preparation process is free ofammonia nitrogen wastewater.
Buprofenzin process for the preparation of
-
Paragraph 0010; 0013-0015; 0018-0020; 0023-0025; 0028-0030, (2019/11/28)
The invention relates to a preparation method of buprofezin, which comprises the following steps of actinism, chlorination, synthesis and distillation crystallization. The actinism step employs dioxane or chloroform, the chlorination step employs dioxane or N-chloromethyl-N-phenyl carbamyl chloride as a solvent; and the synthesis step employs organic amine to participate in condensation. According to the invention, dioxane, chloroform or N-chloromethyl-N-phenyl carbamyl chloride are employed as the solvents, so that good dissolving performance is provided to the raw material, and the dioxane, chloroform or N-chloromethyl-N-phenyl carbamyl chloride has little solubility to HCL and is in favor of reaction, the production yield can reach as high as more than 82%; organic amine is employed for replacing ammonium bicarbonate as alkali for synthesis reaction, and can be recovered so that the discharge of ammonia nitrogen in waste water is reduced.
69327-76-0 Process route
-
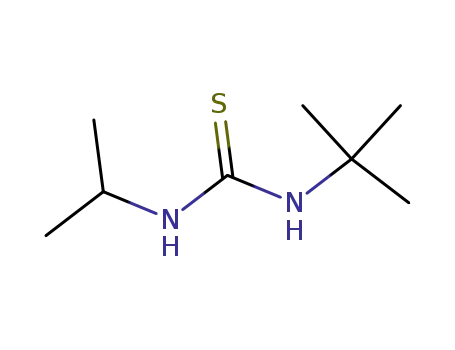
- 52599-24-3
N-tert-butyl-N'-isopropylthiourea

-
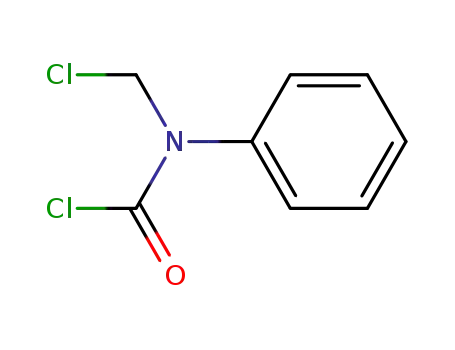
- 52123-54-3
N-Chloromethyl-N-phenylcarbamoyl chloride

-
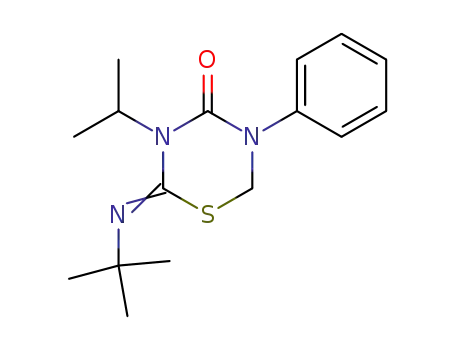
- 69327-76-0,953030-84-7
buprofezin
| Conditions | Yield |
|---|---|
|
N-Chloromethyl-N-phenylcarbamoyl chloride; With dipotassium peroxodisulfate; 3,6-dimethyl-3,6-dihydroperoxy-4,5-dioxaoctane; at 40 - 45 ℃; Flow reactor; Large scale;
N-tert-butyl-N'-isopropylthiourea; With tetradecyltrimethylammonium chloride; calcium hydroxide; at 15 - 50 ℃; Reagent/catalyst; Temperature; Flow reactor; Large scale;
|
92.3% |
|
With sodium acetate; In water; toluene; at 30 - 45 ℃; for 2h; Temperature; Reagent/catalyst;
|
87% |
|
With sodium hydrogencarbonate; In water; chlorobenzene; at 0 - 30 ℃; for 5.5h; Green chemistry;
|
|
|
With ammonia; In 1,4-dioxane; at 10 - 40 ℃; for 5h; Reagent/catalyst;
|
-
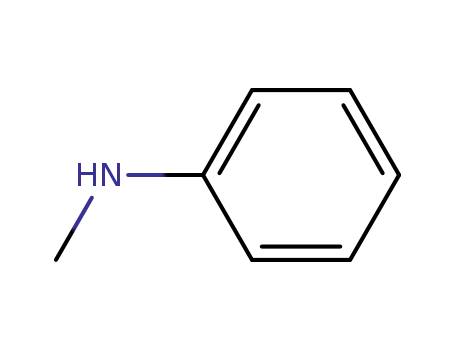
- 100-61-8
N-methylaniline

-

- 69327-76-0,953030-84-7
buprofezin
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: chlorine / chlorobenzene / 40 °C / Green chemistry
2: chlorobenzene / Green chemistry
3: sodium hydrogencarbonate / water; chlorobenzene / 5.5 h / 0 - 30 °C / Green chemistry
With chlorine; sodium hydrogencarbonate; In water; chlorobenzene;
|
|
|
Multi-step reaction with 3 steps
1: chloroform / 15 - 50 °C
2: 2,2'-azobis(isobutyronitrile); chlorine / 55 °C
3: ammonia / 1,4-dioxane / 5 h / 10 - 40 °C
With 2,2'-azobis(isobutyronitrile); ammonia; chlorine; In 1,4-dioxane; chloroform;
|
|
|
Multi-step reaction with 3 steps
1.1: n-butyl formate / 50 - 60 °C / Flow reactor; Large scale
2.1: hydrogenchloride / 50 - 60 °C / Flow reactor; Large scale
3.1: 3,6-dimethyl-3,6-dihydroperoxy-4,5-dioxaoctane; dipotassium peroxodisulfate / 40 - 45 °C / Flow reactor; Large scale
3.2: 15 - 50 °C / Flow reactor; Large scale
With hydrogenchloride; dipotassium peroxodisulfate; n-butyl formate; 3,6-dimethyl-3,6-dihydroperoxy-4,5-dioxaoctane;
|
69327-76-0 Upstream products
-
590-42-1
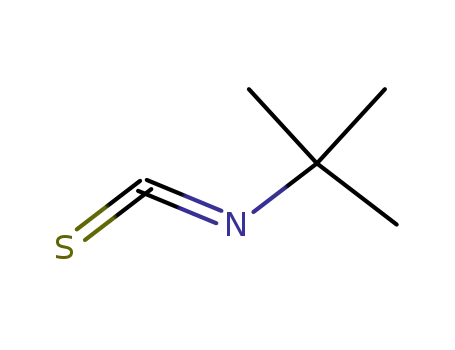
tert-butyl isothiocyanate
-
52599-24-3

N-tert-butyl-N'-isopropylthiourea
-
52123-54-3

N-Chloromethyl-N-phenylcarbamoyl chloride
-
100-61-8
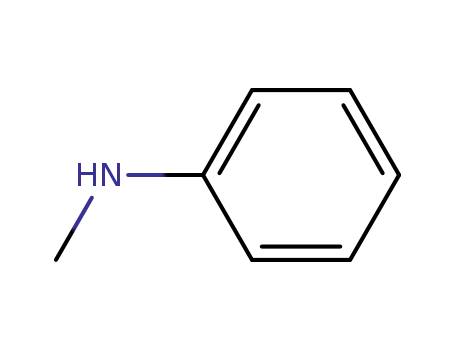
N-methylaniline