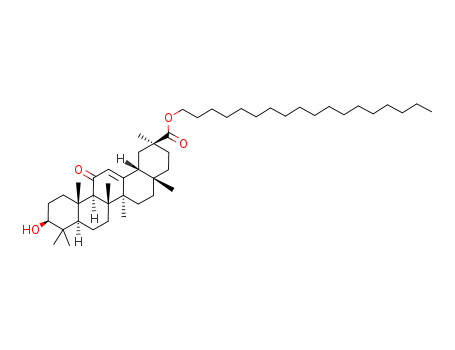
13832-70-7
- Product Name:stearyl glycyrrhetinate
- Molecular Formula:C48H82 O4
- Purity:95%
- Molecular Weight:
Product Details
Packing:1k/bag
Appearance:white powder
pd_productuse:cometics raw material
Chinese Trustworthy Factory Supply Reliable Best Quality 13832-70-7 with Safe Shipping
- Molecular Formula:C48H82 O4
- Molecular Weight:723.16
- Vapor Pressure:0mmHg at 25°C
- Melting Point:70-77 °C
- Boiling Point:732.5°Cat760mmHg
- PKA:15.11±0.70(Predicted)
- Flash Point:198.8°C
- PSA:63.60000
- Density:1.01g/cm3
- LogP:13.13270
Octadecyl 3-hydroxy-11-oxoolean-12-en-29-oate(Cas 13832-70-7) Usage
|
General Description |
Stearyl glycyrrhetinate is a stearic acid ester of glycyrrhetinic acid, generally used as a flavoring agent in commercially available cosmetic products. |
InChI:InChI=1/C48H82O4/c1-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23-24-33-52-42(51)45(5)30-29-44(4)31-32-47(7)36(37(44)35-45)34-38(49)41-46(6)27-26-40(50)43(2,3)39(46)25-28-48(41,47)8/h34,37,39-41,50H,9-33,35H2,1-8H3/t37-,39-,40-,41+,44+,45-,46-,47+,48+/m0/s1
13832-70-7 Relevant articles
Method for synthesizing octadecyl glycyrrhetinate
-
Page/Page column 5-6, (2020/05/14)
The invention discloses a method for syn...
Synthetic studies on the relationship between anti-HIV activities and micelle forming abilities of various alkylated glycyrrhetinate diglycoside sodium sulfates and related compounds
Saito,Furumoto,Ochiai,Hosono,Hoshino,Haraguchi,Ikeda,Shimada
, p. 365 - 381 (2007/10/03)
Sodium sulfates 11-14, 29-32, 35 and 37 ...
13832-70-7 Process route
-
- 471-53-4
enoxolone

-
- 112-89-0
1-Bromooctadecane

-
- 13832-70-7
glycyrrhetinyl stearate
| Conditions | Yield |
|---|---|
|
enoxolone; With sodium hydroxide; In ethanol; water; at 50 ℃; for 0.5h; Ionic liquid;
1-Bromooctadecane; for 4h; Temperature; Reflux;
|
-
- 112-92-5
1-octadecanol

-
- 471-53-4
enoxolone

-
- 13832-70-7
glycyrrhetinyl stearate
| Conditions | Yield |
|---|---|
|
With pyridine; 2-chloro-1,3-dimethyl imidazolium chloride; for 24h; Ambient temperature;
|
74.4% |
13832-70-7 Upstream products
-
112-92-5
1-octadecanol
-
471-53-4
enoxolone
-
135459-36-8
30-stearyl glycyrrhizin
-
112-89-0
1-Bromooctadecane
13832-70-7 Downstream products
-
1367192-03-7
O-(2-O-acetyl-3,4,6-tri-O-benzyl-α-D-mannopyranosyl) stearyl glycyrrhetinate

