1405-86-3
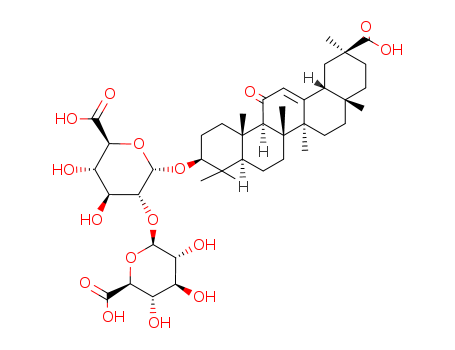
- Product Name:Glycyrrhizic acid
- Molecular Formula:C42H62O16
- Purity:99%
- Molecular Weight:
Product Details
Appearance:White powder
Buy High Quality 1405-86-3 In Bulk Supply with Cheapest Price and Efficient Delivery
- Molecular Formula:C42H62O16
- Molecular Weight:822.945
- Appearance/Colour:White powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:220ºC decomposes
- Refractive Index:61 ° (C=1.5, EtOH)
- Boiling Point:971.4 °C at 760 mmHg
- PKA:2.76±0.70(Predicted)
- Flash Point:288.1 °C
- PSA:267.04000
- Density:1.43 g/cm3
- LogP:2.24560
Glycyrrhizic acid(Cas 1405-86-3) Usage
|
|
|
|
Definition |
ChEBI: A triterpenoid saponin that is the glucosiduronide derivative of 3beta-hydroxy-11-oxoolean-12-en-30-oic acid. |
|
Safety Profile |
Poison by intravenous route. Moderately toxic by ingestion and intraperitoneal routes. Human systemic effects by ingestion: somnolence and changes in the metabolism of phosphorus, When heated to decomposition it emits acrid smoke and irritating fumes. |
InChI:InChI=1/C41H60O16.3H3N/c1-37(2)21-8-11-40(5)18-7-10-38(3)13-14-39(4,36(52)53)16-19(38)17(18)15-20(42)31(40)41(21,6)12-9-22(37)54-34-27(47)25(45)28(30(57-34)33(50)51)55-35-26(46)23(43)24(44)29(56-35)32(48)49;;;/h15,18-19,21-31,34-35,43-47H,7-14,16H2,1-6H3,(H,48,49)(H,50,51)(H,52,53);3*1H3/t18?,19-,21?,22-,23-,24-,25+,26+,27+,28-,29-,30-,31?,34+,35+,38-,39-,40-,41-;;;/m0.../s1
1405-86-3 Relevant articles
Syntheses of glycyrrhetic acid α-diglycosides and enol α-glycosides
Saito,Sumita,Kanda,Sasaki
, p. 1016 - 1027 (1994)
Glycyrrhetinate α-monoglycoside derivati...
A method for the preparation of trans-glycyrrhizic acid
-
Paragraph 0018; 0021; 0024, (2019/07/04)
The invention takes the market glycyrrhi...
METHOD FOR PRODUCING GLYCYRRHIZINIC ACID AND GALACTURO GLYCYRRHIZINIC ACID, AND INTERMEDIATE USED FOR THE PRODUCTION METHOD
-
Paragraph 0039; 0045, (2018/07/28)
PROBLEM TO BE SOLVED: To provide a synth...
Methyl isoglycyrrhizinate refining method
-
Paragraph 0016, (2018/04/02)
According to the present invention, a me...
Acyclovir transdermal delivery system
-
, (2008/06/13)
A transdermal formulation for providing ...
1405-86-3 Process route
-
-
diammonium glycyrrhizinate

-
- 1405-86-3
glycyrrhizin

-
-
18α-glycyrrhizic acid
| Conditions | Yield |
|---|---|
|
With sulfuric acid; In methanol; at 0 ℃; pH=3; Overall yield = 98 g;
|
56.25 % de |
-
-
C45H68O15

-
- 1405-86-3
glycyrrhizin
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; for 2h; Reflux;
|
8.5 g |
1405-86-3 Upstream products
-
30430-55-8
trimethyl glycyrrhizate
-
149553-79-7
C42H62O12
-
60192-37-2
C55H78O21
-
68340-31-8
2-O-(β-D-glucopyranosyl)-β-D-glucopyranosyl-11-oxoolean-12-en-30-oate
1405-86-3 Downstream products
-
6556-12-3
D-glucuronic acid
-
471-53-4
enoxolone
-
489-91-8
D-glucuronic acid
-
147218-57-3
18β-glycyrrhetinic acid α-D-glucuronic acid

